
Welcome to Amanda's NICU Education




Hi! My name is Amanda. I'm a NICU nurse, Clinical Nurse Specialist, NICU Educator... basically your NICU BFF. If you want to talk NICU, I'm here for you! I love everything about NICU nursing and I'm eager to learn and share my knowledge with all my NICU friends.
I have been a NICU nurse since 2009 I am currently a Clinical Nurse Specialist in a Level IV NICU in Los Angeles.
I am passionate about educating the next generation of NICU nurses. I share my knowledge through platforms such as Instagram and Facebook and am excited to have you here on my website!
Click on the button below to sign up for my newsletter filled with NICU education and tips for all experience levels.

Not very many people love taking tests but as a self-acclaimed "forever student" who has taken (and passed) five different certification exams I am no longer afraid of tests! "Way to brag", you might be thinking but I want to help YOU pass your certification exam too!
Introducing Amanda's RNC-NIC Success digital course - your ultimate study companion!
Gain unlimited, on-demand access for life, ensuring you're primed to ace your certification exam.
I'm here to help you succeed and I can't wait for you to share with me that you PASSED the RNC-NIC EXAM!!!









Cyanotic Heart Defects: The Terrible T's!
The Terrible T's
Happy week four of heart month! Let’s wrap up this month by reviewing the cyanotic congenital heart defects. There’s a little trick I learned to help remember some of them:
1️⃣ Truncus Arteriosus (one common artery)
2️⃣ Transposition of the Great Arteries (the two great arteries are transposed)
3️⃣ Tricuspid Atresia (tri = three)
4️⃣ Tetralogy of Fallot (four defects in ToF)
5️⃣ TAPVR (five letters in TAPVR)
Let’s quickly review each of the defects and some key points we should know when we are caring for babies affected by them in the NICU.
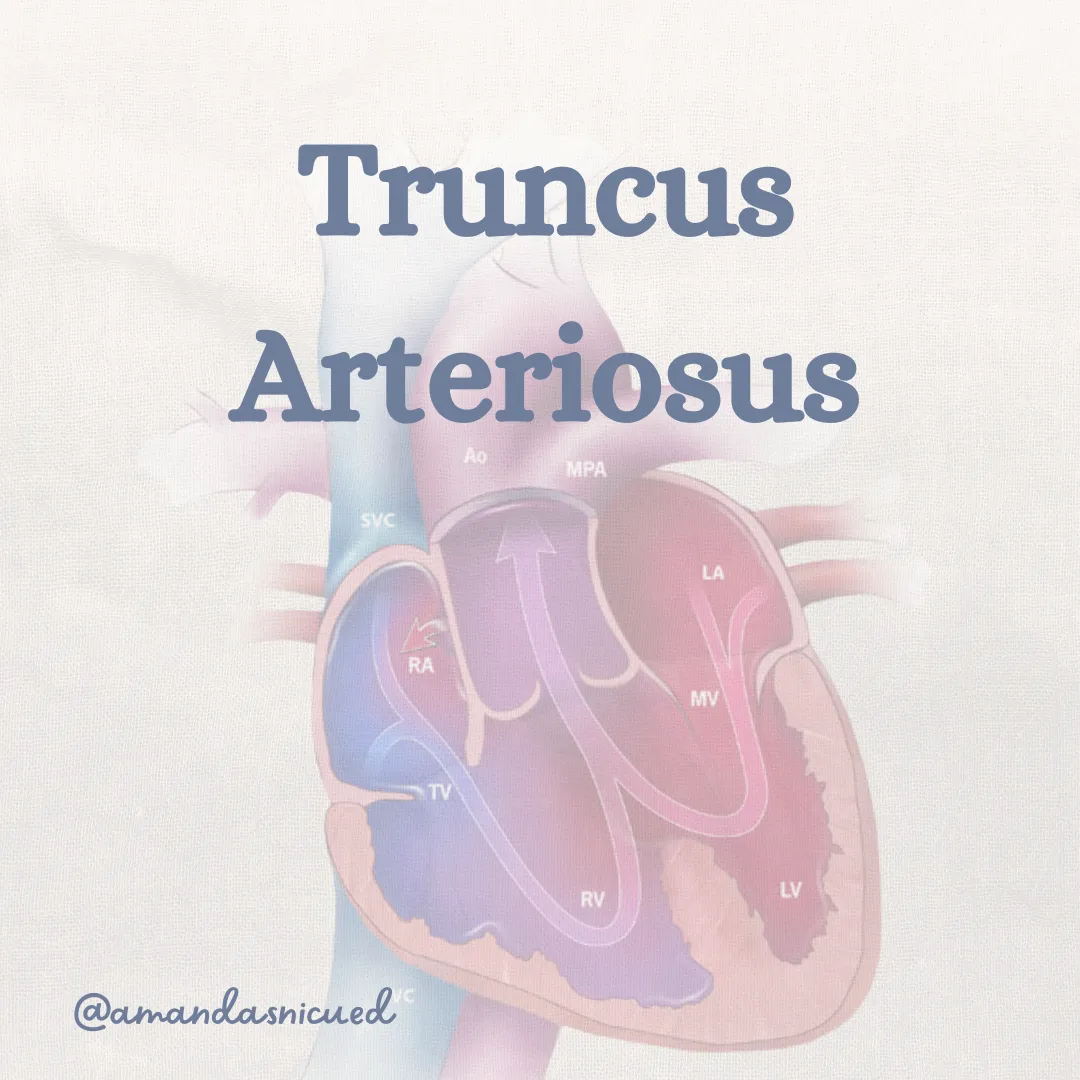
Truncus Arteriosus
Truncus arteriosus is a rare congenital heart defect where the aorta and pulmonary artery arise as one large artery instead of two separate arteries resulting in a single common arterial trunk.

The single arterial trunk overrides a large ventricular septal defect (VSD), allowing blood from both ventricles to mix. This condition is complicated with symptoms of congestive heart failure (CHF) in the newborn period after increased pulmonary vascular resistance declines.
Clinical manifestations of truncus arteriosus include mild cyanosis, poor feeding, tachypnea, and failure to thrive. Signs of heart failure are also seen and include diaphoresis, difficulty breathing, poor weight gain, and hepatomegaly.
Diagnosis of truncus arteriosus is made using echocardiography, which can visualize the single arterial trunk and associated cardiac abnormalities. Coronary anomalies also frequently occur in patients with truncus arteriosus and must be evaluated. Cardiac catheterization is used, only when the full anatomy is unable to be clearly defined on echo.
Preoperative care of the patient with truncus arteriosus involves close monitoring of vital signs, allowing oxygen saturations in the 70s to 80s. Supplemental oxygen administration can worsen pulmonary edema. Genetic evaluation for 22q11 deletion should (DiGeorge Syndrome) also be completed preoperatively as conotruncal defects are associated with this syndrome.
Management of truncus arteriosus involves surgical repair within the neonatal period. The goal of surgery is to create separate pulmonary and systemic circulations by closing the VSD and reconstructing the pulmonary arteries. In some cases, a temporary surgical shunt may be placed to increase pulmonary blood flow prior to definitive repair.
Long-term outcomes for infants with truncus arteriosus depend on the severity of associated cardiac anomalies and the success of surgical intervention. The major late complication after truncus arteriosus repair is obstruction or stenosis of the conduit. Follow-up with a pediatric cardiologist is necessary to monitor cardiac function and address any complications.

Transposition of the Great Arteries
D-transposition of the great arteries (TGA) is a common serious heart condition. In TGA, the aorta and pulmonary artery are switched, leading to parallel circulations that necessitate blood shunting for survival.
Mixing of oxygenated and deoxygenated blood only happens with associated lesions (e.g., PFO, ASD, PDA, or VSD). The extent of mixing depends on factors such as the number, size, and position of shunts or lesions, pressure differentials, and changes in vascular resistance.
During assessment, you may observe varying degrees of cyanosis based on intracardiac mixing. Mild cyanosis may occur with a significant VSD or PDA, while profound cyanosis is seen with an intact ventricular septum or closing PDA. Administration of additional oxygen doesn't necessarily improve cyanosis. Aim for pulse oximetry saturations above 75%, and provide oxygen and ventilation support as needed.
In babies with TGA, reverse differential cyanosis may occur, where the right hand's saturation is lower than that in the foot. Initially, saturations equalize, both being low, due to elevated PVR. This is distinctly different from what is observed in babies with PPHN and is an important observation.
Stressors like crying, feeding, or exposure to cold can worsen cyanosis. If PaO2 at rest is below 35 mm Hg or persistent metabolic acidosis is present, suspect inadequate intracardiac mixing. These infants may require interventions to improve intracardiac mixing such as a PGE infusion or a balloon septostomy.
A balloon septostomy involves inserting a catheter across the PFO, enlarging a balloon, and pulling the catheter through to create an ASD, enhancing mixing between circulations.
X-rays in babies with TGA may appear normal, show increased pulmonary vascularity, or have an "egg on a string" appearance due to a narrow mediastinum from a small thymus and artery alignment. Echocardiograms are diagnostic, revealing the transposed arteries, open fetal shunts, and existing lesions (ASD, VSD).
Medical management involves PGE infusion and intermittent arterial blood gas analysis to monitor for metabolic acidosis, indicating insufficient mixing of oxygenated and deoxygenated blood. Provide supplemental oxygen as needed while monitoring pulse oximetry. Intubation and conventional ventilation may be necessary for treating PGE-associated apnea. Apnea and fever are common side effects of PGE.
Surgical treatment involves the "arterial switch" procedure, typically performed in the first week of life. Surgical complications include dysrhythmias, myocardial ischemia or infarction, and pulmonary supravalvular stenosis. The prognosis after a successful arterial switch operation is favorable, but lifelong cardiology follow-up is necessary to monitor common longer-term issues.
Tricuspid Atresia
Tricuspid atresia is characterized by the absence or severe underdevelopment of the tricuspid valve. The tricuspid valve separates the right atrium from the right ventricle resulting in a "single ventricle" physiology. Sometimes a VSD is present, which allows the RV to be closer to normal size. However if the ventricular septum is intact the RV is hypoplastic.
About 30-50% of infants with tricuspid atresia have other anomalies (eg TGA, COA).
Blood returning from the body to the heart is unable to flow through the tricuspid valve into the right ventricle as it normally would. Instead, blood is redirected through an alternate pathway, such as an atrial septal defect (ASD) or patent foramen ovale (PFO), to reach the left side of the heart and mix with oxygenated blood before being pumped out to the body.
Tricuspid atresia presents with cyanosis shortly after birth. It is crucial that the ductus arteriosus remains open for blood to circulate to the lungs therefore these infants require a PGE infusion. Infants with tricuspid atresia may also exhibit symptoms of CHF, such as tachypnea, poor feeding, and failure to thrive.
Diagnosis of tricuspid atresia is made using echocardiography, which can visualize the absence or severe underdevelopment of the tricuspid valve and associated cardiac abnormalities.
Management of tricuspid atresia often involves staged surgical interventions to improve blood flow and oxygenation. This may include procedures such as the creation of a systemic-to-pulmonary artery shunt (Blalock Taussig shunt) to increase blood flow to the lungs, followed by the bidirectional Glenn and Fontan procedure to redirect blood flow directly to the pulmonary arteries and bypass the right ventricle. Any associated defects will also be repaired.
Long-term outcomes for individuals with tricuspid atresia is guarded and depends on the severity of the defect, associated cardiac anomalies, and the success of surgical interventions. Regular follow-up with a pediatric cardiologist is essential to monitor cardiac function, growth, and development. Heart transplant may eventually be needed.
Tetralogy of Fallot
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart defect. It comprises four components: a VSD, overriding of the ascending aorta, obstruction of the right ventricular outflow tract, and right ventricular hypertrophy.

The terms 'pink tet' and 'blue tet' refer to the degree of right ventricular outflow tract obstruction. A 'pink tet' describes minimal obstruction, resulting in little to no cyanosis, while a 'blue tet' indicates severe obstruction, leading to significant cyanosis. In severe cases, where pulmonary blood flow is markedly reduced, the ductus arteriosus plays a crucial role in redirecting blood flow to the lungs, necessitating PGE therapy and neonatal surgery.
Most infants with TOF fall between these extremes and have sufficient pulmonary blood flow without CHF symptoms. The predominant intracardiac shunt in TOF is usually right-to-left, resulting in varying degrees of cyanosis depending on the level of shunting and pulmonary blood flow.
During assessment, a loud harsh systolic murmur may be heard at the mid- to upper left sternal border, which diminishes or disappears during hypercyanotic spells. Anemia can exacerbate hypoxemia in these cases, therefore it is important to monitor hemoglobin levels.
Echocardiography is diagnostic, providing details about the severity and location of pulmonary stenosis and the size of the VSD.
Immediate medical intervention is generally unnecessary for stable neonates with TOF. However, those with severe right ventricular outflow tract obstruction may require PGE infusion to maintain ductal patency.
Infants with TOF are susceptible to hypercyanotic spells, characterized by severe and persistent cyanosis, irritability, pallor, tachypnea, and potential loss of consciousness. These spells warrant prompt medical attention and, in some cases, emergent surgery if conservative management fails.
Total correction of TOF typically involves intracardiac repair with patch closure of the VSD and relief of the right ventricular outflow tract obstruction. Surgical repair is usually performed electively within the first year of life. Long-term follow-up is essential due to the risk of pulmonary valve dysfunction and associated complications
Total Anomalous Pulmonary Venous Return
Total Anomalous Pulmonary Venous Return (TAPVR) is a rare defect where all four pulmonary veins, which normally return oxygenated blood from the lungs to the left atrium, connect abnormally to the right atrium or other systemic veins. This prevents oxygenated blood from draining into the left side of the heart and out to the body.
In TAPVR, oxygenated blood returning from the lungs is redirected into the right side of the heart, leading to mixing of oxygenated and deoxygenated blood. This causes cyanosis and inadequate oxygen delivery to the body's tissues.
There are different types of TAPVR based on the specific anatomical abnormalities of the pulmonary venous connections. These include supracardiac (most common), cardiac, infracardiac, and mixed types, each with its unique pattern of venous drainage.
Supracardiac TAPVR: pulmonary veins drain into the SVC
Cardiac TAPVR: pulmonary veins drain into the RA or coronary sinus
Infracardiac TAPVR: pulmonary veins drain inferiorly through the diaphragm, connecting through the portal venous system of IVC
Mixed lesions: at least two of the types described occurs in the same baby.
Clinical manifestations of TAPVR depend on the degree of pulmonary venous obstruction and associated cardiac anomalies. Infants with unobstructed TAPVR will have symptoms of left to right shunting, eventually causing signs of CHF (eg tachypnea, tachycardia, and failure to thrive). They are typically identified after the neonatal period due to heart failure and can be managed on diuretics until elective TAPVR repair.
The presence of an obstruction results in rapid deterioration. Obstructed TAPVR causes pulmonary congestion leading to decreased pulmonary blood flow and pulmonary hypertension, exacerbating cyanosis, metabolic acidosis, and eventually circulatory collapse. Medical management prior to surgery will involve stabilization, optimization of oxygen delivery, and prevention of pulmonary hypertensive crisis. Mechanical ventilation, inotropic support, PGE infusion are utilized until emergent surgery can be performed.
Diagnosis of TAPVR is made using echocardiography, which can visualize the abnormal connections of the pulmonary veins and associated cardiac abnormalities. Chest X-Ray may demonstrate pulmonary venous congestion without cardiomegaly, or increased pulmonary vascularity with cardiomegaly depending on the presence of obstruction.
For patients with TAPVR, correction in the newborn period is associated with low mortality. The development of postoperative pulmonary vein stenosis is the most serious long-term complication. Follow-up with a pediatric cardiologist is essential to monitor cardiac function, growth, and development



That was a great review of the "terrible T's"! I didn't want this newsletter to be too long so I will be sending you a bonus newsletter tomorrow reviewing the remaining cyanotic defects (HLHS, Pulmonary Atresia, Double Outlet RV, and Ebsteins anomaly).
Wishing you all the best!
Amanda xoxo
Missed my other newsletters? Click here to read them!
Let's Study Together! Join my Certification Course
References:
Swanson, T. & Erickson, L. (2021). Cardiovascular Disease and Surgical Interventions from Merenstein and Gardner's Handbook of Neonatal Intensive Care. Elsevier
Murthy, R. Nigro, J., Karamlou, T. (2019). Tricuspid Atresia from Critical Heart Diseases in Infants and Children. Elsevier
St. Louis, J., Molitor-Kirsch, E., Shah, S., O'Brien, J. (2019). Total Anomalous Pulmonary Venous Return from Critical Heart Diseases in Infants and Children. Elsevier
Jaggers, J., & Cole, C., (2019). Truncus Arteriosus from Critical Heart Diseases in Infants and Children. Elsevier
Sadowski, S., & Verklan, T. (2021). Cardiovascular disorders in Core Curriculum for Neonatal Intensive Care Nursing (6th Ed). Elsevier

Copyright © {{right_now.year}} {{location.name}}, All rights reserved.
Our mailing address is:
{{location.email}}

December 2023 Certification Review Webinar
NICU Certification Review



Ready to kickstart your journey to becoming a certified NICU nurse?
Look no further!
Grab my FREE E-Book packed with essential study and test-taking strategies for the RNC-NIC.
In the E-Book I give you the resources you need including the link to access the candidate guide, several types of books to study from, some of my favorite strategies, an outline of the content you should review, and a blank calendar for you to make your study plan!
Frequently Asked Questions About the RNC-NIC exam

What is the RNC-NIC?
The RNC-NIC is a competency-based exam that tests the specialty knowledge of nurses in the United States & Canada who care for critically ill newborns and their families.
The RNC-NICU is a nationally recognized certification that recognizes the registered nurse for their specialty knowledge and skill.

Who can take the RNC-NIC exam?
Nurses can take this exam after a minimum of two years experience in the NICU caring for critically ill newborns and their families.

Which books should I use?
I'm glad you asked! There are many excellent books to help you prepare for the RNC-NIC, I gathered ande describe each of them for you in my FREE e-book.
Is there a course to help me study?
Yes! Many hospitals host their own certification course and there are a few online courses. See my RNC-NIC test taking tips E Book for more information
What happens if I don't pass the exam?
If you don't pass the exam on your first try you can try again after 90 days. You will have to reapply after 90 days and pay a retest fee. There is no limit to the number of times you can take the exam (however a candidate can only sit for the exam twice per year).
Can I make more money if I take the RNC-NIC exam and get certified?
Yes! Many hospitals provide a raise or a bonus for nurses with specialty certifications. Hospitals also typically hire at a higher base salary when nurses have a certification.

Find me @amandasnicued on these channels or Email me
hey nurses don't miss out
© Copyright 2024. AmandasNICUEd. All rights reserved. | Terms & Conditions | Privacy Policy Contact: [email protected]

