
Welcome to Amanda's NICU Education




Hi! My name is Amanda. I'm a NICU nurse, Clinical Nurse Specialist, NICU Educator... basically your NICU BFF. If you want to talk NICU, I'm here for you! I love everything about NICU nursing and I'm eager to learn and share my knowledge with all my NICU friends.
I have been a NICU nurse since 2009 I am currently a Clinical Nurse Specialist in a Level IV NICU in Los Angeles.
I am passionate about educating the next generation of NICU nurses. I share my knowledge through platforms such as Instagram and Facebook and am excited to have you here on my website!
Click on the button below to sign up for my newsletter filled with NICU education and tips for all experience levels.

Not very many people love taking tests but as a self-acclaimed "forever student" who has taken (and passed) five different certification exams I am no longer afraid of tests! "Way to brag", you might be thinking but I want to help YOU pass your certification exam too!
Introducing Amanda's RNC-NIC Success digital course - your ultimate study companion!
Gain unlimited, on-demand access for life, ensuring you're primed to ace your certification exam.
I'm here to help you succeed and I can't wait for you to share with me that you PASSED the RNC-NIC EXAM!!!









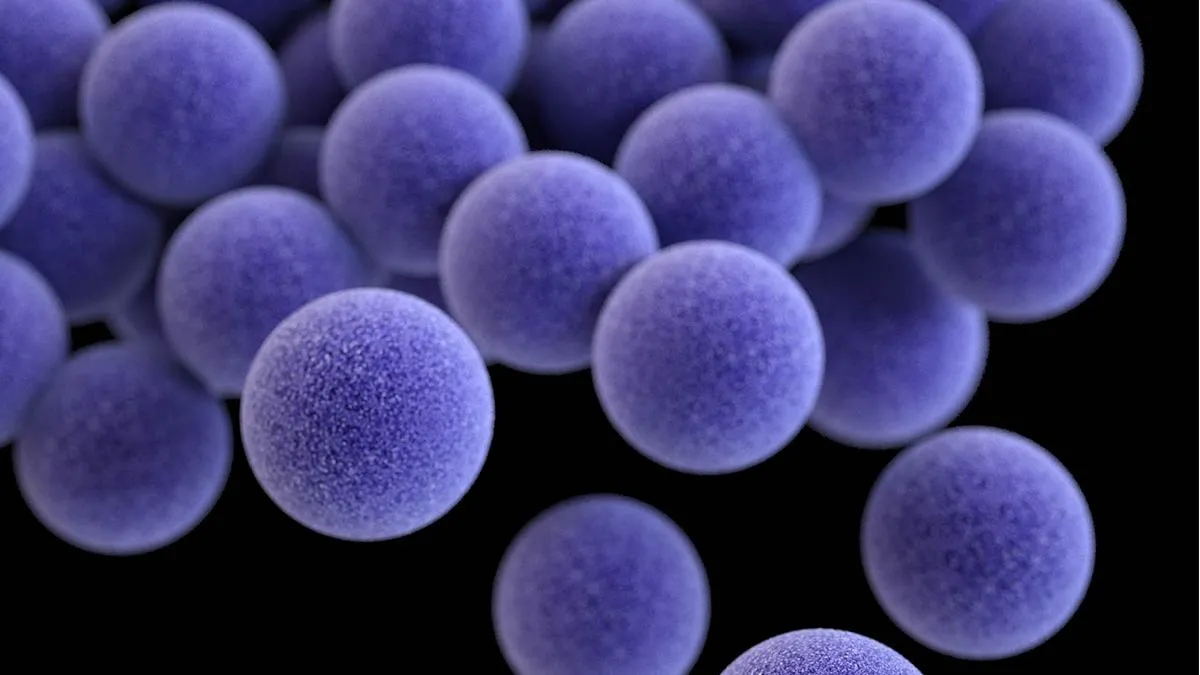
MSSA
A colleague reached out to me a week or so ago requesting information about MSSA, their NICU was experiencing an outbreak and the nurses felt like they needed more information. At a similar time, other colleagues were discussing screening protocols and what the standard is. Staph aureus seems to be a hot topic so let's review it together!
As NICU nurses, we must recognize that MSSA infections represent a common cause of late-onset sepsis in our most vulnerable patients. While MRSA often captures our attention due to its antibiotic resistance, MSSA infections actually occur more frequently in NICUs and can lead to equally devastating outcomes. Together, let’s explore the critical aspects of S. aureus prevention, recognition, and management, with particular emphasis on innovative approaches like maternal skin-to-skin contact that show promising results for decolonization without compromising the developing microbiome. Make sure you read to the end to hear about that interesting RCT!
What is Staphylococcus Aureus? 🩺
Staphylococcus aureus is a gram-positive bacterium that exists as part of the normal flora on the skin and mucous membranes of healthy individuals. In the NICU environment, this seemingly harmless commensal bacteria can rapidly transform into a dangerous pathogen, particularly in neonates requiring intensive care. The organism's ability to cause bloodstream infections, pneumonia, skin and soft tissue infections, and severe systemic illness makes it a major problem for our most vulnerable patient population.
Transmission in the NICU occurs primarily through direct contact, contaminated equipment, or healthcare personnel, which emphasizes why strict infection prevention measures form the cornerstone of our defense strategy. Understanding these transmission patterns helps us recognize that every interaction with our patients carries potential risk, making our role in prevention absolutely critical.

Center for Disease Control & Prevention, 2024
MSSA vs MRSA
The fundamental difference between Methicillin-Resistant Staphylococcus aureus (MRSA) and Methicillin-Sensitive Staphylococcus aureus (MSSA) lies in their antibiotic susceptibility profiles, though both can cause equally severe infections in neonates.
MRSA demonstrates resistance to methicillin and other beta-lactam antibiotics including ampicillin, oxacillin, cephalosporins, and carbapenems due to the presence of the mecA gene.
This resistance necessitates alternative treatment approaches, typically involving vancomycin as first-line therapy.
MSSA remains sensitive to methicillin and other beta-lactam antibiotics, making it treatable with penicillinase-resistant penicillins such as nafcillin or oxacillin.
However, this sensitivity should not diminish our concern about MSSA infections.
While MRSA has earned a reputation for being more difficult to treat, MSSA infections can be equally severe in neonates, particularly those with compromised immune systems or multiple risk factors.
The clinical significance of this distinction extends beyond treatment choices. MSSA's sensitivity to common antibiotics might create a false sense of security, potentially leading to delayed recognition or less aggressive prevention measures. As NICU nurses, we must maintain the same level of vigilance for both strains, understanding that the patient outcomes can be equally devastating regardless of antibiotic sensitivity.
Identifying High-Risk Patients
The identification of patients at highest risk for S. aureus infection requires a comprehensive understanding of multiple interconnected factors. Prematurity and very low birth weight represent the most significant risk factors, as these infants possess immature immune systems that cannot mount effective responses to bacterial challenges. The administration of antibacterial therapy, while often necessary, can disrupt the microbiome resulting in dysbiosis and creating opportunities for S. aureus colonization and subsequent infection.
Invasive devices present ongoing risks throughout the NICU stay. Central lines and peripheral IVs create direct pathways for bacterial entry into the bloodstream, while endotracheal tubes and mechanical ventilation can facilitate respiratory tract colonization and pneumonia. Additionally, S. aureus produces a biofilm that adheres to these devices, making treatment difficult.
Skin breakdown from immaturity, prolonged hospitalization, frequent procedures, or medical device pressure creates breaches in the primary defense barrier are another important risk factor. Each day of hospitalization increases cumulative risk, as does each invasive procedure, creating a complex risk profile that requires continuous reassessment and vigilant monitoring.
Screening Approaches and Considerations
Routine screening for S. aureus has become common practice in many NICUs as healthcare teams attempt to reduce morbidity and mortality through early identification and intervention. However, the optimal approach to screening remains an area of ongoing debate and research. The body sites screened and frequency of screening vary significantly among institutions, reflecting the lack of universal consensus on best practices.
The Centers for Disease Control and Prevention recommends screening the anterior nares at minimum, recognizing this as a primary site of S. aureus colonization. However, many NICUs expand their screening to include additional sites such as the umbilicus and rectum, acknowledging that colonization patterns in neonates may differ from those in older populations. The optimal target population for conducting active surveillance testing should be determined in collaboration with the epidemiology team at each institution, taking into account local epidemiology, resources, and risk factors.
The timing and frequency of screening present additional considerations. Some units screen all admissions, while others focus on high-risk populations or outbreak situations. The decision about screening frequency must balance the potential benefits of early detection against the stress and discomfort of repeated procedures, particularly in extremely preterm infants where every handling event carries significance.
Video on S Aureus from Osmosis
Prevention Strategies:
Fundamental Infection Control Measures
The foundation of S. aureus prevention rests on meticulous adherence to fundamental infection control principles. Hand hygiene remains the single most effective intervention for preventing transmission, yet achieving consistent compliance requires ongoing education, monitoring, and reinforcement. The technique must be thorough, the timing appropriate, and the compliance universal among all staff members, regardless of their role or seniority.
Standard Precautions must be implemented consistently for all patients with MSSA, while Contact Precautions for any patient with confirmed MRSA. Many NICUs also utilize Contact Precautions with outborn infants until screening cultures have resulted, follow your hospitals policies. The implementation of these precautions extends beyond simply donning personal protective equipment; it requires understanding the rationale, following proper procedures, and maintaining vigilance throughout patient care activities.
Patient isolation and cohorting strategies provide additional layers of protection when appropriately implemented. The decision to isolate patients must consider both infection control benefits and potential negative impacts on patient care, family bonding, and staff efficiency. When isolation is necessary, it must be implemented thoroughly and consistently, with clear communication among all team members about the requirements and rationale.
Environmental and Personnel Interventions
Environmental controls play a crucial role in preventing S. aureus transmission within the NICU. Rigorous cleaning and disinfection protocols must be established and followed consistently, with particular attention to high-touch surfaces and shared equipment.
Staffing considerations impact infection prevention in multiple ways. Appropriate nurse-to-patient ratios allow for more thorough hand hygiene and infection control practices, while staff cohorting during outbreaks can help limit transmission. However, these interventions must be implemented thoughtfully, considering their impact on staff scheduling, patient care continuity, and overall unit operations.
Healthcare personnel interventions may include screening programs to identify colonized staff members, decolonization protocols when indicated, and work restrictions for personnel who pose transmission risks. These interventions require careful consideration of privacy rights, employment implications, and the balance between infection control benefits and practical workforce management.

Clinical Recognition and Assessment
MSSA represents a common cause of late-onset sepsis, particularly among preterm infants with indwelling devices. Late-onset sepsis, defined as infection occurring after 72 hours of life, often presents with subtle and nonspecific signs that can be easily overlooked or attributed to other causes. The challenge for NICU nurses lies in recognizing these early warning signs and advocating for appropriate evaluation and intervention.
Temperature instability may manifest as hypothermia, hyperthermia, or temperature fluctuations that deviate from the infant's baseline patterns.
Respiratory changes including apnea, bradycardia, and desaturation events may represent early signs of sepsis, particularly when they occur with increased frequency or severity.
Changes in work of breathing or respiratory deterioration should prompt immediate assessment, as should alterations in ventilator requirements or oxygen needs.
Behavioral changes often provide early clues to developing sepsis.
Lethargy, decreased responsiveness, or changes in feeding tolerance may indicate the onset of systemic illness.
Gastrointestinal symptoms including feeding intolerance, abdominal distension, or changes in stool patterns can also signal developing sepsis, particularly in conjunction with other concerning signs.
Local Signs and Complications
The assessment for S. aureus infection must include careful examination of all potential sites of infection. Erythema, swelling, or drainage at catheter insertion sites may indicate local infection that could progress to systemic illness. Surgical sites require ongoing assessment for signs of infection, including changes in wound appearance, increased drainage, or delayed healing.
Early identification and treatment are essential to prevent the progression to serious complications. Persistent bacteremia can lead to seeding of distant sites, resulting in conditions such as meningitis, endocarditis, or osteomyelitis. These complications carry significant morbidity and mortality risks, making early recognition and aggressive treatment paramount.
The long-term neurodevelopmental impacts of untreated or inadequately treated sepsis underscore the importance of maintaining high clinical suspicion and advocating for appropriate diagnostic evaluation when clinical signs suggest possible infection. The nonspecific nature of early sepsis signs requires nurses to trust their clinical instincts and communicate concerns effectively with the healthcare team.
Treatment and Management Strategies
Supportive Care Principles
The management of infants with confirmed S. aureus infections requires comprehensive supportive care that addresses the multisystem effects of sepsis while facilitating recovery.
Respiratory support may need to be escalated, with careful attention to ventilator settings, oxygenation requirements, and the potential need for advanced respiratory interventions.
Cardiovascular monitoring becomes crucial as sepsis can lead to shock, requiring aggressive fluid resuscitation and potentially vasoactive medication support.
The provision of appropriate IV fluids requires careful balance, considering the infant's fluid needs, electrolyte balance, and cardiovascular status.
Close monitoring for complications becomes essential, with particular attention to signs of persistent bacteremia, seeding of distant sites, or development of shock or other life-threatening complications.
Nutritional support may need modification during acute illness, with consideration given to enteral feeding tolerance and the potential need for parenteral nutrition.
The coordination of care among multiple subspecialists may become necessary, particularly if complications develop that require surgical intervention or specialized management.
Antimicrobial Therapy and Decolonization
Decolonization may be performed when an infant (or family) is colonized, but not infected. Current decolonization strategies present both opportunities and challenges. Mupirocin, applied topically to the anterior nares, can be effective but presents technical challenges in very preterm infants due to the small size of the nasal passages and concerns about systemic absorption following intranasal administration. Chlorhexidine bathing represents another approach, but the risk of adverse skin reactions, including chemical burns in extremely premature infants, requires careful consideration.
The emergence of resistance following universal treatment approaches has raised concerns about the widespread use of decolonization agents. From a microbiome protection perspective, the decolonization of infants who are not colonized with S. aureus raises questions about the impact on the developing microbiome and the potential for unintended consequences.
Infections with S aureus require treatment with antimicrobials. The choice of antimicrobial therapy depends on the susceptibility profile of the isolated organism and the severity of the clinical presentation. For MRSA infections, vancomycin typically serves as first-line therapy, with careful monitoring of drug levels and renal function. MSSA infections can be treated with beta-lactam antibiotics such as nafcillin or oxacillin, which often provide superior efficacy compared to vancomycin for sensitive organisms.

Innovative Approaches: Maternal Skin-to-Skin Contact
Research from Brazil has revealed an innovative and promising approach to S. aureus decolonization that deserves significant attention. A randomized controlled trial explored whether maternal skin-to-skin contact could help decolonize preterm infants colonized with multidrug-resistant staphylococci, yielding remarkable results that challenge traditional approaches to decolonization.
The study involved infants who received skin-to-skin contact twice daily for seven days with their non-colonized parent, compared to a control group that did not receive this intervention. The results found infants who received skin-to-skin contact achieved decolonization rates of 52.8%, compared to only 22.4% in the control group. Perhaps most interestingly, this improvement occurred without the use of antibiotics or topical agents, avoiding the potential complications and resistance issues associated with traditional decolonization methods.
The proposed mechanism behind this success involves bacterial interference, where the mother's protective microbiome gradually replaces the resistant flora colonizing the infant. This natural process respects the developing microbiome while providing effective decolonization, offering a safe, low-cost strategy that simultaneously promotes bonding and reduces multidrug-resistant colonization.
Clinical Implications and Implementation
The implications of this research extend far beyond simple decolonization strategies. In an era where we increasingly recognize the importance of the developing microbiome and the potential negative impacts of broad-spectrum antimicrobial interventions, maternal skin-to-skin contact offers a natural, physiologic approach to infection prevention that aligns with our understanding of optimal neonatal care.
The implementation of increased skin-to-skin contact opportunities requires careful consideration of individual patient factors, family readiness, and clinical stability. However, for clinically stable infants with appropriate family support, this intervention offers multiple benefits including improved bonding, enhanced physiologic stability, and now demonstrated decolonization effects.
As advocates for our patients and families, NICU nurses are uniquely positioned to promote and facilitate skin-to-skin contact opportunities. This involves educating families about the benefits, assessing readiness for skin-to-skin contact, and advocating with the healthcare team for appropriate opportunities when clinically indicated.
Let's Reflect...
The translation of knowledge into practice requires concrete actions that can be implemented immediately and sustained over time. Begin by reviewing your unit's current S. aureus screening and isolation protocols, ensuring you understand the rationale and can implement them consistently. Assess your own hand hygiene technique and work to encourage compliance among your colleagues, recognizing that this fundamental intervention remains our most powerful tool for prevention.
Advocate for appropriate skin-to-skin contact opportunities for your patients, educating families about the benefits and working with the healthcare team to identify suitable candidates. Maintain ongoing vigilance in monitoring high-risk patients for subtle signs of infection, trusting your clinical instincts and advocating for appropriate evaluation when concerns arise.
Stay current with your facility's antibiotic protocols and participate actively in unit-based infection prevention initiatives. Your role as a bedside nurse places you in a unique position to identify risks, implement preventive measures, and advocate for your patients' safety and well-being.
Our role extends beyond simple task completion. We educate families, vigilantly monitor our patients and advocate for them. Our work can literally save lives. This is all through your commitment to excellence in infection prevention, early recognition, and comprehensive care. Thank you for serving as the front-line defense against S. aureus infections in our most vulnerable patients.
You are appreciated,
Amanda
Missed my other newsletters? Click here to read them!
References:
Centers for Disease Control and Prevention (CDC) guidelines on Staphylococcus aureus
Lamy Filho, F., de Sousa, S. H., Freitas, I. J., Lamy, Z. C., Simões, V. M., da Silva, A. A., & Barbieri, M. A. (2015). Effect of maternal skin-to-skin contact on decolonization of Methicillin-Oxacillin-Resistant Staphylococcus in neonatal intensive care units: a randomized controlled trial. BMC pregnancy and childbirth, 15, 63.
Rooney, C. M., Lancaster, R., McKechnie, L., & Sethi, K. (2024). Staphylococcal aureus outbreaks in neonatal intensive care units: strategies, nuances, and lessons learned from the frontline. Antimicrobial stewardship & healthcare epidemiology, 4(1), e70.
Nelson, M. U., & Gallagher, P. G. (2012). Methicillin-resistant Staphylococcus aureus in the neonatal intensive care unit. Seminars in perinatology, 36(6), 424–430.
Mahieu, L. Engelen, A., Hensels, E., Van Damme, K., & Matheeussen, V. (2024). Surveillance on methicillin-sensitive Staphylococcus aureus colonization and infection in a neonatal intensive care unit. Journal of Hospital Infection, 143, 195-202.


December 2023 Certification Review Webinar
NICU Certification Review



Ready to kickstart your journey to becoming a certified NICU nurse?
Look no further!
Grab my FREE E-Book packed with essential study and test-taking strategies for the RNC-NIC.
In the E-Book I give you the resources you need including the link to access the candidate guide, several types of books to study from, some of my favorite strategies, an outline of the content you should review, and a blank calendar for you to make your study plan!
Frequently Asked Questions About the RNC-NIC exam

What is the RNC-NIC?
The RNC-NIC is a competency-based exam that tests the specialty knowledge of nurses in the United States & Canada who care for critically ill newborns and their families.
The RNC-NICU is a nationally recognized certification that recognizes the registered nurse for their specialty knowledge and skill.

Who can take the RNC-NIC exam?
Nurses can take this exam after a minimum of two years experience in the NICU caring for critically ill newborns and their families.

Which books should I use?
I'm glad you asked! There are many excellent books to help you prepare for the RNC-NIC, I gathered ande describe each of them for you in my FREE e-book.
Is there a course to help me study?
Yes! Many hospitals host their own certification course and there are a few online courses. See my RNC-NIC test taking tips E Book for more information
What happens if I don't pass the exam?
If you don't pass the exam on your first try you can try again after 90 days. You will have to reapply after 90 days and pay a retest fee. There is no limit to the number of times you can take the exam (however a candidate can only sit for the exam twice per year).
Can I make more money if I take the RNC-NIC exam and get certified?
Yes! Many hospitals provide a raise or a bonus for nurses with specialty certifications. Hospitals also typically hire at a higher base salary when nurses have a certification.

Find me @amandasnicued on these channels or Email me
hey nurses don't miss out
© Copyright 2024. AmandasNICUEd. All rights reserved. | Terms & Conditions | Privacy Policy Contact: [email protected]

