
Welcome to Amanda's NICU Education




Hi! My name is Amanda. I'm a NICU nurse, Clinical Nurse Specialist, NICU Educator... basically your NICU BFF. If you want to talk NICU, I'm here for you! I love everything about NICU nursing and I'm eager to learn and share my knowledge with all my NICU friends.
I have been a NICU nurse since 2009 I am currently a Clinical Nurse Specialist in a Level IV NICU in Los Angeles.
I am passionate about educating the next generation of NICU nurses. I share my knowledge through platforms such as Instagram and Facebook and am excited to have you here on my website!
Click on the button below to sign up for my newsletter filled with NICU education and tips for all experience levels.

Not very many people love taking tests but as a self-acclaimed "forever student" who has taken (and passed) five different certification exams I am no longer afraid of tests! "Way to brag", you might be thinking but I want to help YOU pass your certification exam too!
Introducing Amanda's RNC-NIC Success digital course - your ultimate study companion!
Gain unlimited, on-demand access for life, ensuring you're primed to ace your certification exam.
I'm here to help you succeed and I can't wait for you to share with me that you PASSED the RNC-NIC EXAM!!!









Cyanotic Heart Defects: Ebstein Left 5 Terrible T Pods
What about the other cyanotic defects?
Do you recall the other cyanotic heart lesions? Well, I've been putting on my thinking cap, and I'm thrilled to unveil a nifty phrase I concocted to help us remember them all:
'Ebstein left 5 terrible T Pods'
Let's break it down!
Ebstein Anomaly
Hypoplastic Left Heart Syndrome
5 Terrible T's
Pulmonary Atresia
Double Outlet Right Ventricle
Isn't that catchy? With this phrase, we can effortlessly recall all eight cyanotic congenital heart defects. Pretty cool, right?
Now, let's keep the excitement rolling!
Today, we're delving deeper into four fascinating heart conditions: Ebstein's Anomaly, Hypoplastic Left Heart Syndrome (HLHS), Pulmonary Atresia, and Double Outlet Right Ventricle (DORV).
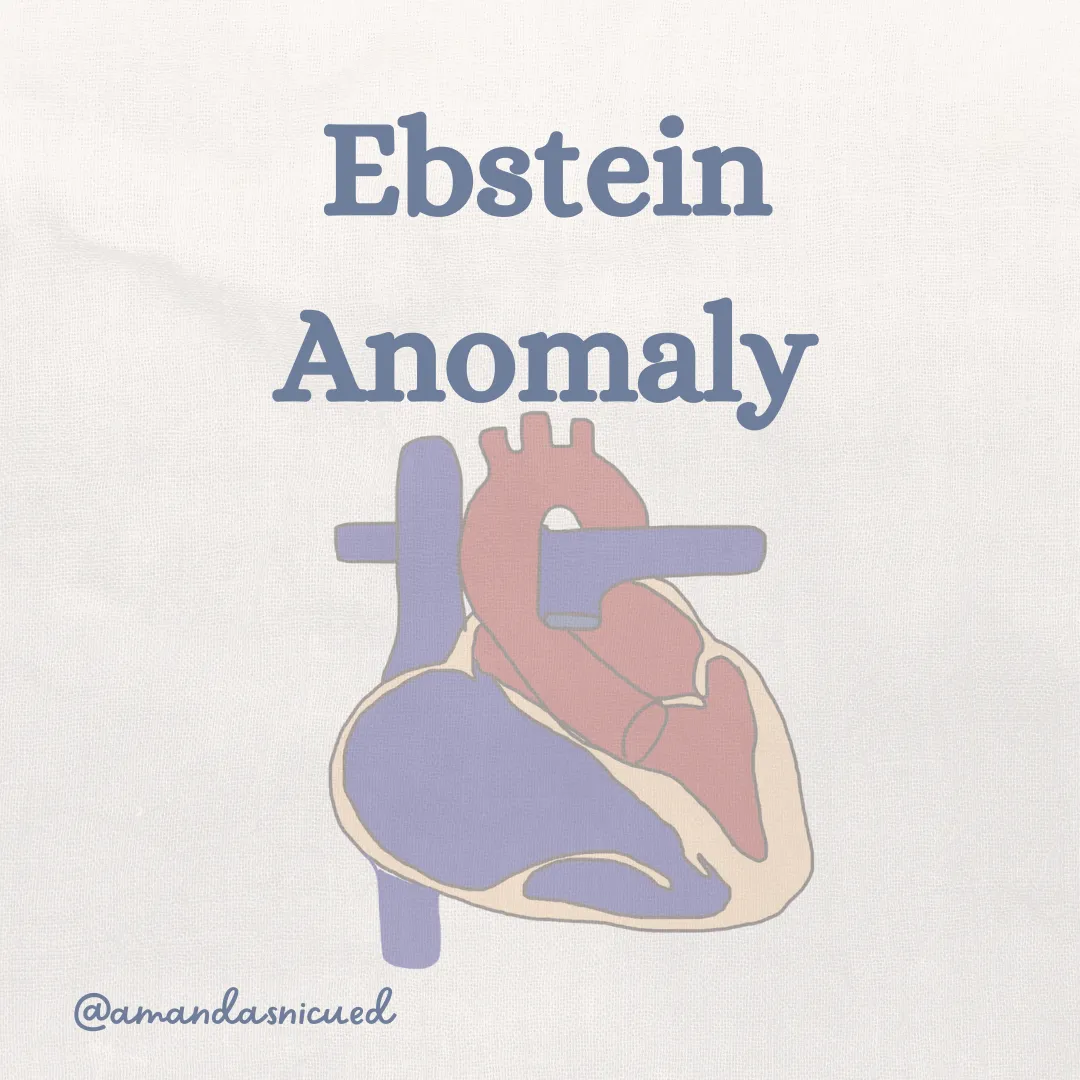
Ebstein Anomaly
Ebstein's Anomaly is a rare congenital heart defect that affects the tricuspid valve, which separates the right atrium from the right ventricle. In this condition, the tricuspid valve is abnormally positioned lower in the right ventricle, leading to a displacement of the valve leaflets and a varying degree of malfunction.

This displacement results in an enlarged "atrialized" portion of the right ventricle. The dysfunctional tricuspid valve results in a RV that is small and ineffective. Blood is unable to be ejected up into the pulmonary artery due to the decreased pumping ability of the RV and the RV outflow tract obstruction. This results in severe cardiomegaly with lung hypoplasia, cyanosis, and shunting of blood across the ASD/PFO.
The clinical presentation of Ebstein's anomaly can vary depending on the severity of the condition. Infants with severe forms of Ebstein's anomaly may present shortly after birth with cyanosis, respiratory distress, and heart failure.
On chest x-ray, patients with Ebstein's anomaly have a large "box-like" heart. I have always remembered Ebstein's being referred to as "the biggest heart you've ever seen" because the heart takes up almost the entire chest. Diagnosis of Ebstein's anomaly is made using echocardiography, which can visualize the displacement and dysfunction of the tricuspid valve.
The management of Ebstein's anomaly depends on the severity of the condition. Patients who are relatively stable require supplemental oxygen and prostaglandin infusion and close monitoring for adequacy of cardiac output and oxygen saturations. Unstable patients will require intubation with mechanical ventilation, sedation, paralytics, PGE, and inotropes prior to surgery. Milrinone is the preferred inotrope and catecholamines should be avoided as they can predispose a tachyarrythmia. The risk of mortality is high when surgical repair is done in neonates, therefore it is preferred to medically manage as long as possible.
The prognosis for individuals with Ebstein's anomaly can vary depending on the severity of the condition and the presence of associated cardiac abnormalities. Typically the earlier patients demonstrate symptoms of Ebstein the more severe and poorer prognosis. However, with advancements in medical and surgical treatments, many individuals with Ebstein's anomaly can lead fulfilling lives with appropriate management and follow-up care.

Hypoplastic Left Heart Syndrome (HLHS)
Hypoplastic left heart syndrome (HLHS) is a complex congenital heart defect characterized by underdevelopment of the left side of the heart, including the left ventricle (LV) and associated structures. HLHS encompasses a spectrum of cardiac malformations, but key features include a small or atretic LV, abnormal mitral and aortic valves, and hypoplasia of the ascending aorta and aortic arch.
Coarctation of the aorta may be present in as many as 75% of patients. The severity of the malformations can vary, leading to different subtypes of HLHS.
In HLHS, the right ventricle (RV) must support both the pulmonary and systemic circulations due to the underdeveloped left side of the heart. Survival relies on a patent ductus arteriosus to maintain systemic perfusion and an atrial septal defect for adequate mixing of oxygenated and deoxygenated blood. The relative distribution of RV output to the systemic and pulmonary circulations depends on various factors, including pulmonary vascular resistance and the size of the ASD.
Similarly, the presentation of HLHS varies depending on factors such as the size of the ASD and the patency of the ductus arteriosus. Common clinical features include cyanosis, respiratory distress, diminished peripheral pulses, and hepatomegaly. Diagnosis is made through echocardiographic imaging, either prenatally or postnatally, although other diagnostic tests such as pulse oximetry and electrocardiography may also be used.
HLHS is medically managed by ensuring sufficient mixing of oxygenated and deoxygenated blood and optimizing ventricular function. This includes initiating PGE to ensure ductal patency and systemic circulation. Medical support to prevent CHF is often needed, including mechanical ventilation, diuretics, and inotropes. A balloon atrial septostomy may also be needed in infants with a restrictive atrial septum.
Surgical repair is performed using a staged approach. The first surgery is the "Norwood" procedure and involves creating a common atrial chamber for both systemic and pulmonary return by removing the atrial septum and reconstructing the aortic arch. The PDA is ligated and a Blalock-Taussig shunt is created connecting the RV to pulmonary circulation. The Norwood procedure is the only surgery performed in the neonatal period. The second surgery is the bidirectional Glenn procedure at ~3-6 months. The final surgery is the Fontan at around 3-4 years.
HLHS carries higher risk of morbidity and mortality compared to other congenital heart diseases. Additionally, long term morbidity risks include neurodevelopment delay, arrhythmias, hypertension, growth restriction, and heart failure.
Check Out this great video describing the Norwood procedure
Pulmonary Atresia
Pulmonary atresia involves complete obstruction of the pulmonic valve, leading to a hypoplastic right ventricle and tricuspid valve. However, if a VSD is present, the right ventricle may be of adequate size. In pulmonary atresia, the deoxygenated blood is unable to travel up the into the pulmonary artery because it is obstructed. Instead the blood bypasses the obstructed pulmonic valve by crossing the foramen ovale into the left atrium, then into the left ventricle, and finally out the aorta. Blood flow to the lungs occurs entirely through a left-to-right shunt at the ductus arteriosus, which is can be small and tortuous. As the ductus arteriosus closes, severe hypoxemia develops. Understandably PGE is needed to keep the ductus arteriosus open for survival. Clinical manifestations of pulmonary atresia include cyanosis at birth, progressing to intense cyanosis, tachypnea, and metabolic acidosis as the PDA begins closes.
Diagnosis is made by echocardiography. Chest X-ray shows increased heart size with decreased pulmonary markings.
Initial treatment of pulmonary atresia includes PGE to maintain ductal patency as well as fluid, oxygen, and correction of metabolic acidosis. Digitalis and diuretics are used to manage CHF.
Cardiac catheterization may involve balloon atrial septostomy to improve shunting across the PFO. Balloon valvuloplasty, with or without stent placement, is used in small neonates and infants. Surgical interventions may include valvotomy for mild right ventricular hypertrophy or a Blalock–Taussig shunt to provide systemic-to-pulmonary shunting in severe cases.
Long-term management may involve completion of the Fontan procedure or heart–lung transplant in severe cases.
Long-term complications include arrhythmias, CHF, infective endocarditis, brain abscesses, delayed growth, and sudden death. Long-term management and monitoring are essential for addressing potential complications and ensuring the best possible quality of life for affected individuals.

Diagnosis is made by echocardiography. Chest X-ray shows increased heart size with decreased pulmonary markings.
Initial treatment of pulmonary atresia includes PGE to maintain ductal patency as well as fluid, oxygen, and correction of metabolic acidosis. Digitalis and diuretics are used to manage CHF.
Cardiac catheterization may involve balloon atrial septostomy to improve shunting across the PFO. Balloon valvuloplasty, with or without stent placement, is used in small neonates and infants. Surgical interventions may include valvotomy for mild right ventricular hypertrophy or a Blalock–Taussig shunt to provide systemic-to-pulmonary shunting in severe cases.
Long-term management may involve completion of the Fontan procedure or heart–lung transplant in severe cases.
Long-term complications include arrhythmias, CHF, infective endocarditis, brain abscesses, delayed growth, and sudden death. Long-term management and monitoring are essential for addressing potential complications and ensuring the best possible quality of life for affected individuals.
Double Outlet Right Ventricle
Double outlet right ventricle (DORV) is a complex congenital heart defect where both the pulmonary artery and the aorta connect to the right ventricle. This condition results in abnormal circulation and varying degrees of oxygenation in the blood.

Types of DORV can range in complexity with different arrangements and a VSD (which is often present) can have a varying degree of locations. Pulmonary stenosis can also be present. Additionally, coronary anomalies occur is about 50% of patients with DORV further complicating this defect.
The clinical manifestations of DORV can vary widely depending on the associated heart defects and the degree of mixing between oxygenated and deoxygenated blood. Infants with DORV with a VSD and no pulmonary stenosis can have pulmonary overcirculation progressing to CHF within the first week of life. Common signs may include cyanosis, shortness of breath, tachypnea, poor feeding, and failure to thrive. Chest X-Ray may reveal cardiomegaly and increased pulmonary markings
Infants with TOF type DORV (subaortic VSD with Pulmonary stenosis) can be asymptomatic initially or with mild cyanosis as the PS balances systemic and pulmoary blood flow. The PDA may be required to keep adequate blood flow to the lungs therefore PGE may be initiated.
Patients with TGA-type DORV (aorta is positioned more anteriorly and PA more posteriorly with a subpulmonary VSD) can demonstrate hypoxia from inadequate mixing manifested as cyanosis, tachypnea, and metabolic acidosis. Presurgical management is focused on improving mixing of oxygenated and deoxygenated blood by initiating PGE and balloon septostomy.
The type of DORV is diagnosed using echocardiogram.
Surgical repair of DORV is often repaired around 3 months of age, unless excessive pulmonary blood flow, CHF, of constricted pulmonary blood flow necessitates earlier interventions.
The prognosis for children with DORV varies depending on the severity of the heart defects, associated complications, and the timing and success of treatment. Children with DORV require close follow up with a cardiologist to monitor for complications such as baffle or RVOT obstruction which may require intervention.

Wow! What a great way to finish up February! I hope that review was super helpful. Email me and let me know what you thought, I always love hearing from you!
Wishing you all the best!
Amanda xoxo
Missed my other newsletters? Click here to read them!
Let's Study Together! Join my Certification Course
References:
Swanson, T. & Erickson, L. (2021). Cardiovascular Disease and Surgical Interventions from Merenstein and Gardner's Handbook of Neonatal Intensive Care. Elsevier
Shusheel Kumar, T.K., Knott-Craig, C (2019). Ebstein Anomaly from Critical Heart Diseases in Infants and Children. Elsevier
Connolly, H., Yasir Qureshi, M, Dearani, J. (2023). Ebstein anomaly: Management and prognosis. Retrieved from www.up-to-date.com
Bichell, D. (2019) Double Outlet Right Ventricle from Critical Heart Diseases in Infants and Children. Elsevier
Image Credits:

December 2023 Certification Review Webinar
NICU Certification Review



Ready to kickstart your journey to becoming a certified NICU nurse?
Look no further!
Grab my FREE E-Book packed with essential study and test-taking strategies for the RNC-NIC.
In the E-Book I give you the resources you need including the link to access the candidate guide, several types of books to study from, some of my favorite strategies, an outline of the content you should review, and a blank calendar for you to make your study plan!
Frequently Asked Questions About the RNC-NIC exam

What is the RNC-NIC?
The RNC-NIC is a competency-based exam that tests the specialty knowledge of nurses in the United States & Canada who care for critically ill newborns and their families.
The RNC-NICU is a nationally recognized certification that recognizes the registered nurse for their specialty knowledge and skill.

Who can take the RNC-NIC exam?
Nurses can take this exam after a minimum of two years experience in the NICU caring for critically ill newborns and their families.

Which books should I use?
I'm glad you asked! There are many excellent books to help you prepare for the RNC-NIC, I gathered ande describe each of them for you in my FREE e-book.
Is there a course to help me study?
Yes! Many hospitals host their own certification course and there are a few online courses. See my RNC-NIC test taking tips E Book for more information
What happens if I don't pass the exam?
If you don't pass the exam on your first try you can try again after 90 days. You will have to reapply after 90 days and pay a retest fee. There is no limit to the number of times you can take the exam (however a candidate can only sit for the exam twice per year).
Can I make more money if I take the RNC-NIC exam and get certified?
Yes! Many hospitals provide a raise or a bonus for nurses with specialty certifications. Hospitals also typically hire at a higher base salary when nurses have a certification.

Find me @amandasnicued on these channels or Email me
hey nurses don't miss out
© Copyright 2024. AmandasNICUEd. All rights reserved. | Terms & Conditions | Privacy Policy Contact: [email protected]

